Conventional and Quantitative Electroencephalography in Psychiatry
John R. Hughes, M.D., Ph.D. and E. Roy John, Ph.D.
Received April 9, 1998; revised accepted May 22, 1998. From the Department of Neurology, University of Illinois School of Medicine, Chicago, Illinois; Department of Psychiatry, New York University Medical Center, New York, New York; and Nathan S. Kline Institute for Psychiatric Research, Orangeburg, New York. Address correspondence to Dr. John, Brain Research Laboratories, New York University Medical Center, 550 First Avenue, New York, NY, 10016.
SOURCE: J Neuropsychiatry Clin Neurosci 11:190-208, May 1999
© 1999 American Psychiatric Press, Inc.

|
ABSTRACT |
Electrical activity of each brain region is homeostatically regulated, resulting in predictable frequency composition of the background EEG. Replicated normative databases have established that the EEG power spectrum is independent of ethnic background. Artifact-free EEG evaluated relative to such norms displays few deviant values in healthy, normally functioning individuals. In subjects with psychiatric disorders, high proportions of abnormal findings have been reported with good concordance and high specificity and sensitivity across numerous studies, distinctive within a wide variety of disorders and often contributing to differential diagnosis and selection of treatment. New three-dimensional QEEG imaging methods offer an economical alternative to other functional brain imaging modalities.
Key Words: Electroencephalography

|
INTRODUCTION |
New brain imaging technologies are yielding information about structural or functional abnormalities in patients with various psychiatric disorders. These imaging modalities include magnetic resonance imaging; positron emission tomography of regional cerebral metabolic rate, regional cerebral blood flow, and radioligand binding to receptors of neurotransmitters; single-photon emission computed tomography; functional magnetic resonance imaging; magnetoencephalography; quantitative electroencephalography (QEEG) and event-related potentials (ERP); topographic QEEG and statistical probability mapping; and low-resolution electromagnetic tomography. Evidence from these brain imaging methods has unequivocally established that "mental illness" has definite correlates with brain dysfunction. QEEG and ERP methods afford the psychiatric practitioner a set of noninvasive tools that are capable of quantitatively assessing resting and evoked activity of the brain with sensitivity and temporal resolution superior to those of any other imaging method.
Conditions such as anxiety disorder, depression, dementia, obsessive-compulsive disorder, schizophrenia, learning disabilities, and attention deficit disorder with and without hyperactivity are now understood to involve interactions between brain dysfunctions or altered neuroanatomical structure and environmental influences. Medications that profoundly alter the availability of neurotransmitters and affect a hypothesized pathophysiology are routinely prescribed by psychiatric practitioners. Nonetheless, little or no attempt is made in most cases, even in the treatment-resistant patient, to use biological assessment methods to select a treatment, to evaluate its physiological effect, and to demonstrate its efficacy objectively. Relevant biological measurements may become invaluable adjuncts for the selection and evaluation of treatment and may minimize false starts, decrease severity and shorten duration of symptoms, and markedly reduce costs. In this era, it will become increasingly essential to demonstrate the need for treatment and to substantiate its efficacy.

|
EEG AND QEEG |
Of all the imaging modalities, the greatest body of replicated evidence regarding pathophysiological concomitants of psychiatric and developmental disorders has been provided by EEG and QEEG studies. Electrophysiological assessment is also the most practical of these methods, using relatively simple, inexpensive, compact equipment readily accommodated by clinics, hospitals, or private offices. QEEG analytical algorithms are widely available from commercial sources, and workshops with continuing medical education accreditation in collection, analysis, and interpretation of data are now regularly presented by professional societies as well as equipment manufacturers. However, despite extensive evidence of sensitivity and specificity, the adoption of QEEG by the psychiatric community has been slow. Two major factors may account for this.
First, the numerous reports of abnormalities found in psychiatric patients by visual inspection of the conventional EEG have been regarded as too nonspecific and are usually not included in increasingly compressed curricula. Further, the great majority of recent papers reporting the results of EEG, QEEG, and ERP studies of psychiatric patients have appeared not in psychiatric journals, but rather in specialized electrophysiological or brain research publications.
Second, there has been considerable controversy about the clinical utility of QEEG in position papers published by various professional organizations over the past decade,1- 4 concluding, in the words of one such statement, that "the clinical application of Quantitative EEG is considered to be limited and adjunctive...clinical use...must be an extension of routine EEG."1 These statements cited only a very few published findings in psychiatric disorders, which were frequently grouped with "other disorders" (including tumors, multiple sclerosis, migraine, solvent exposure, and radiation exposure).
During the last decade, more than 500 EEG and QEEG papers have reported well-designed studies, concurring that EEG and QEEG abnormalities are found in a high proportion of psychiatric patients. Individual studies usually include a substantial number of psychiatric patients and normal control subjects, and across all studies within any particular disorder, the overall sample size is very large. An overview of the findings reveals numerous consistent and concordant conventional EEG and QEEG findings among studies within the same DSM diagnoses. Many of these studies have been on never-medicated or unmedicated patients who have been medication free for substantial periods. Statistical significance, specificity, and sensitivity have been high. No comprehensive review of this large body of psychiatrically relevant literature has yet appeared.
This article provides a comprehensive, updated review of how conventional EEG and QEEG can be useful in present clinical psychiatric practice. Other relevant reviews have appeared.5- 8
Basic QEEG Definitions
In QEEG, multichannel recording (usually 19 electrodes at standardized positions) of eyes-closed, resting or "background" EEG are visually edited and a sample of artifact-free data, usually 1 to 2 minutes, is analyzed, using the Fast Fourier Transform (FFT) to quantify the power at each frequency of the EEG averaged across the entire sample, known as the power spectrum. The test-retest replicability of power spectra thus computed has been shown to be highly reproducible in works cited below. The power spectrum of clinical interest is usually considered to extend from about 1 Hz to 20 Hz.
This frequency range has traditionally been separated into 4 wide frequency bands, typically defined as delta (1.5- 3.5 Hz), theta (3.5- 7.5 Hz), alpha (7.5- 12.5 Hz), and beta (12.5- 20 Hz). Results from each electrode can be represented as absolute power in each band (total µV2), relative power in each band (percentage of total power in each channel), coherence (a measure of synchronization between activity in two channels), or symmetry (the ratio of power in each band between a symmetrical pair of electrodes).
Neurophysiological Basis of EEG
Research on the origins of rhythmic brain electrical activity in the various frequency bands indicates that anatomically complex homeostatic systems regulate the EEG power spectrum. Brainstem, thalamic, and cortical processes involving large neuronal populations mediate this regulation, using all the major neurotransmitters.9- 12
Pacemaker neurons distributed throughout the thalamus normally oscillate synchronously in the 7.5- 12.5-Hz frequency range. Efferent projections globally distributed across the cortex produce the rhythmic electrical activity known as the alpha rhythm, which dominates the EEG of an alert healthy person at rest. Nucleus reticularis can hyperpolarize the cell membranes of thalamic neurons by gamma-aminobutyric acid (GABA) release, slowing the dominant alpha rhythm into the lower theta range (3.5- 7.5 Hz) and diminishing sensory throughput to the cortex. Slow delta activity (1.5- 3.5 Hz) is believed to originate in oscillator neurons in deep cortical layers and in the thalamus, normally inhibited by input from the ascending reticular activating system in the midbrain. The faster activity in the beta band (12.5- 20 Hz) is believed to reflect corticocortical and thalamocortical transactions related to specific information processing.
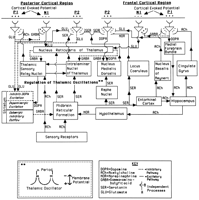
The changes characteristically seen in the disorders reviewed in this paper may be understood by referring to Figure 1. Activation of the mesencephalic reticular formation (MRF) causes inhibition of the nucleus reticularis by cholinergic and serotonergic mediation, which releases the thalamic cells from inhibition by the n. reticularis. The dominant activity of the EEG power spectrum becomes more rapid, with the return of alpha activity and the higher frequency beta activity, and the flow of information through the thalamus to the cortex is facilitated.
View larger version (58K):
|
FIGURE 1. Neurophysiological changes characteristically seen in the disorders reviewed in this paper may reflect disturbances of regulation in this homeostatic systemSee text for a description of this model. |
The cortex can activate n. reticularis directly by glutamatergic pathways to suppress the arrival of information to the cortical level and, by striatal projections, dopamine can inhibit the MRF. Such inhibition of the MRF enables inhibition of thalamic neurons to occur and blocks the flow of sensory information through the thalamus to the cortex.
This model suggests that deficiencies or excesses of any of the neurotransmitters should produce marked departure from the homeostatistically regulated normative EEG spectrum. Such neurotransmitter perturbations are widely believed to make decisive contributions to much psychiatric pathophysiology.
The EEG power spectrum can be reasonably expected to be stable and characteristic for healthy human beings, as a result of homeostatic regulation by these processes, with high specificity probably reflecting our common genetic heritage. It is also reasonable to expect sensitivity in these measures to many dysfunctions believed to be abnormal in some psychiatric disorders. The data support these expectations.
Stability and Specificity of the EEG Power Spectrum as the Basis of QEEG
During the period when the origins of the EEG were being illuminated by studies such as those cited above, the resting EEG power spectra of large samples of healthy, functioning individuals across a wide age range were being studied quantitatively. Initial studies of this sort, using analog methods, showed systematic changes from ages 17 to 64,13 and then from ages 1 to 21,14 in the average power in the delta, theta, alpha, and beta frequency bands. These normative data were soon replicated by use of general-purpose digital computing equipment. Age regression is necessary to correct for maturational effects. Not only were the systematic changes with age confirmed, but no significant differences were found between the EEGs of normally functioning Swedish children and white or black U.S. children.15 Soon thereafter, normally functioning black children in Barbados were found to display the same values of the EEG power spectrum as the U.S. and Swedish groups.
Since then, numerous studies have confirmed the high specificity of normative distributions of power in the delta, theta, alpha, and beta bands. Positive findings different from the normative database in healthy, normally functioning individuals have repeatedly been shown to be within the chance levels, with very high test-retest reliability.16,17 Normative data have been extended to cover the age range from 1 to 95 years for each electrode in the standardized International 10-20 System and have been broadened to include measures of absolute power, relative power, mean frequency, coherence, and symmetry, as well as covariance matrices that quantify normal brain relationships.18- 20 These multivariate composite measures are unique to QEEG; psychiatric disorders rarely entail focal abnormalities.
The independence of the normative QEEG descriptors from cultural and ethnic factors enables objective assessment of brain integrity in persons of any age, origin, or background. This independence and specificity, as well as high replicability, has been established in studies from Barbados, China, Cuba, Germany, Holland, Japan, Korea, Mexico, Netherlands, Sweden, the United States, and Venezuela.17,21- 33 If normative distributions of QEEG measures are made gaussian,15,22 then the incidence of false positive QEEG findings obtained from visually edited, artifact-free resting EEG recordings is at or below the level expected by chance. If one were to require that the QEEG evaluation be performed on two separate samples and that any significant finding deviant at the P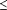 0.05 level be replicated in each of these two samples, the probability that this would occur by chance would be approximately PxP, or 0.05x0.05, or 0.0025. If such a replication were required, false positives would seem rather unlikely. Such a high level of specificity is beyond the confidence level achieved by many routinely used clinical tests, such as mammograms, cervical screenings, or CT brain scans.34
0.05 level be replicated in each of these two samples, the probability that this would occur by chance would be approximately PxP, or 0.05x0.05, or 0.0025. If such a replication were required, false positives would seem rather unlikely. Such a high level of specificity is beyond the confidence level achieved by many routinely used clinical tests, such as mammograms, cervical screenings, or CT brain scans.34
Why Should Psychiatric Patients Have EEG Studies?
In psychiatric diagnostics, physical or neurological conditions must be ruled out before a psychiatric disorder can be diagnosed.35 As many as 64% to 68%2,3 of EEGs in psychiatric patients provide evidence of pathophysiology, and these results have additional utility beyond simply ruling out "organic brain lesions."3 Such electroencephalographic studies may also aid in differential diagnosis, treatment selection, and evaluation. Some longitudinal studies show that initial quantitative EEG profiles may distinguish among patients with the same DSM diagnosis who will respond preferentially to different medications or who will display different evolution of illness.

|
CONVENTIONAL AND QUANTITATIVE EEG STUDIES |
A voluminous literature attests to the robustness of conventional EEG studies and their clinical utility in disorders of brain function. This approach has contributed valuable information for the clinical psychiatrist. This method is essentially based on visual pattern recognition. Over the past 20 years, algorithms for computer pattern recognition, the computer's unsurpassed capacity to measure and calculate, and the availability of normative databases across the human life span have enhanced electroencephalography, supplementing the electroencephalographer's trained eye with a quantitative and objective description of a patient's EEG record (QEEG). Even more powerful are statistical comparisons between numerous measures from the individual patient and those of age-matched normal subjects or of patient subjects having different diagnoses.
These two EEG approaches complement each other: conventional EEG provides reliable diagnostic information, especially sensitive to "organic" or neurological disorders, detecting features of waveshapes, frequency relationships, and transitions of state seldom encountered in the healthy individual. QEEG enables precise comparison of the individual patient's record with normative and psychopathologic patient databases. Across both EEG and QEEG studies, a broad consensus exists on the high proportion of abnormalities found in different psychiatric disorders and often on their electrophysiological profiles. In most of these studies, patients were without psychotropic medication, except for some of the reports evaluating patients with mood disorders or schizophrenia. Most of the chronic patients in those studies had been drug free for some period before evaluation.
Dementias
Electroencephalography is particularly effective but generally underutilized in the evaluation of the confused or delirious patient. The hallmark of delirium usually is the slowing of the background EEG rhythm, to an extent that is positively correlated with the degree of severity of the condition. The one exception is in delirium tremens (DT), which usually shows a normal EEG record with fast rhythms.2,36 If abnormal slow activity is found in the DT condition, consideration should be given to a Wernicke encephalopathy or to a hepatic disorder.37 In the delirium accompanying the neuroleptic malignant syndrome, only a mild diffuse slow wave usually appears.38 When delusional manifestations are prominent, as in organic delusional states, one typically finds increased slow-wave activity over both temporal lobes. Delirium can be differentiated from dementia, and the significant factors are an increased theta activity and increase in delta relative power.39
In organic syndromes showing cognitive deficits such as memory dysfunction, the prevalence of EEG abnormalities is directly related to the degree of cognitive impairment. If clinical impairment is equivocal, the incidence of EEG abnormalities is usually slightly over 40%; with a mild-to-moderate impairment, a 65% incidence is expected.40 The EEG is a moderately sensitive, nonspecific indication of brain dysfunction, clearly useful in the diagnosis of Alzheimer's disease41 and also AIDS dementia,42 with general agreement in the literature that increased slow activity and decreased mean frequency are correlated with cognitive impairment and measures of clinical severity of Alzheimer's dementia.3,43- 45
EEG frequency analysis allows confident detection of excessive slowing, more readily measured and quantified than conventional EEG. QEEG studies in dementia patients are in agreement with conventional EEG findings, reporting increased delta and/or theta power,46- 68 decreased mean frequency,44,61,69,70 decreased beta power,71,72 and decreased occipital dominant frequency.53,58
Many workers regard increased slow activity prior to reduction of alpha power as the earliest electrophysiological indicator appearing in Alzheimer's disease.30,32,47,52,58,63,68,73 The amount of theta activity shows the best correlation with cognitive deterioration11,14,29,31,74- 77 and with clinical outcome in longitudinal follow-up,59,63,68,73,78- 80 although one report found no predictive utility.81 Increased delta appears to be a correlate of severe advanced dementia, occurring subsequent to increased theta.45,60,74,77
In cerebrovascular disease, several EEG frequency parameters are highly correlated with regional blood flow or metabolism. Sensitivity and specificity are high for detection of ischemia-related diffuse or focal impairment.23,82- 88 These studies show sensitivity generally greater than 80%, false-positive rates below 5% to 10%, and correlations of r>0.7 between EEG and blood flow in ischemic and nonischemic regions. EEG slowing is highly correlated with decreased regional cerebral blood flow23,89- 92 or metabolism.10,38,54 QEEG can detect reliable focal features that are missed in the routine EEG and can be quite abnormal even when the CT is still normal, such as in the first 1 to 3 days after stroke or when the degree of ischemia is mild enough to cause dysfunction without infarction.
Alzheimer's dementia and multi-infarct dementia (MID) have been differentiated by evaluating asymmetry of slow activity33,93 and coherence.19,48,94 Multiple studies report accurate discrimination of Alzheimer patients from depressed patients or from normal subjects by use of EEG or QEEG measures of slow activity.37,40,41,44,46,95 Accurate separation of Alzheimer's from frontotemporal dementia (Pick's disease) by use of QEEG has been reported.96
Conclusion:
Routine EEG has long been used to evaluate dementia and encephalopathy unresolved after initial clinical evaluation. There is excellent agreement between conventional and quantitative EEG studies of the dementias. QEEG may be useful in evaluating dementia or encephalopathy if routine EEG studies are not conclusive or if neuroimaging studies are inconclusive or unavailable, as well as in differentiating between Alzheimer's dementia, multi-infarct dementia, depression, and normal aging.
A broad consensus exists across a very large number of EEG and QEEG studies of dementia patients: Both kinds of studies report a diffuse increased delta and/or theta power, with decreased beta power and mean frequency. These features are absent in depression and are focal in multi-infarct dementia, enabling these disorders to be differentiated from Alzheimer's dementia. A good correlation exists between severity of cognitive impairment, clinical outcome, and amount of EEG slowing.
Schizophrenia
Numerous qualitative studies indicate abnormal conventional EEG findings in 20% to 60% of schizophrenic patients.2,97,98 A more specific finding in schizophrenia is a relatively low mean alpha frequency,98,99 although some patients may show a fast alpha rhythm.100 Catatonic patients often present with paroxysmal activity.101
Numerous EEG and QEEG studies of background activity have been performed on carefully evaluated groups of unmedicated as well as medicated schizophrenic patients. Substantial agreement emerges from this body of literature. Deficient alpha power is consistently reported,19,102- 107 as well as altered alpha mean frequency or diminished alpha responsiveness.98,108,109 Numerous studies have reported increased beta activity in schizophrenia.105,110- 113 Neuroleptics typically increase alpha power113- 115 and reduce beta power,116,117 suggesting possible normalization of deviant features by medication.
Increased delta and/or theta activity has also been reported in a large number of studies.101,102,105,106,118- 125 Increased slow activity can apparently result from neuroleptic treatment,126,127 although there are reports of increased delta in patients off medication for several weeks102,105,128 and reduction of delta or theta when medication is resumed.114,125,129 In the elderly schizophrenic patient, an increase in fast theta activity (7- 7.5 Hz) is seen. A decrease in fast alpha (10- 12 Hz) noted on the frontal areas has been called "hypofrontality."100
Clinical relationships reported include 1) correlation between negative symptoms and delta waves in the temporal areas;100 2) positive correlation between degree of QEEG abnormality and degree of clinical improvement,130 raising the question of whether this "degradation" of the EEG is a necessary condition for a clinical improvement with clozapine; and 3) correlation between blink rates, alpha power, and smoking.131 In sleep, a decrease has been found in stages III, IV, and REM, and also in REM latency and sleep continuity.132- 135
Results from a small number of studies are inconsistent with this consensus of a QEEG profile, showing increased delta or theta, decreased alpha, and increased beta in schizophrenia. For example, increased slow activity has not been found by some workers,136,137 and increased alpha100 and decreased beta106,120 have occasionally been reported. Not all of the studies reporting the increased slow activity/decreased alpha/increased beta profile found all of the indicated deviations. This inconsistency plausibly might arise from the coexistence of several subtypes with different QEEG profiles within the population of schizophrenic patients. Observations might depend on the mixture of subtypes within the relatively small samples collected for a particular study.
This heterogeneity has been recently documented in a large sample of medicated, nonmedicated, and never-medicated schizophrenic patients, using cluster analysis based on QEEG variables. Five subtypes were detected, with their QEEG profiles characterized by delta plus theta excess, theta excess, theta plus alpha excess with beta deficit, theta excess, and alpha excess with beta excess.138 Never-medicated patients were classified into three of these subtypes. Schizophrenic patients with QEEG profiles corresponding to some of the groups identified by this cluster analysis have been reported to display differential responses to treatment with haloperidol139or risperidone.140 Additional evidence of heterogeneity in the schizophrenic population has been provided in QEEG studies by other groups.141,142
Findings of asymmetry in schizophrenia have been inconsistent. However, these findings depend upon whether measurements were made over anterior or posterior regions. When the electrode array covered both regions, power was highest over the right hemisphere in anterior regions, but over the left hemisphere in posterior regions.20,120,142 This conclusion was supported by the cluster analysis just cited, in which this asymmetry pattern was found in every frequency band for all five subtypes.138 However, increased amounts of delta activity in the left anterior temporal area100 have been reported to discriminate schizophrenic patients from control subjects.
Increased interhemispheric coherence in anterior regions has been consistently found.121,138,143- 145 In view of the decreased frontal coherence in depressive illness cited below, QEEG separation of schizophrenic patients from bipolar depressed patients may be possible.
Conclusion:
Evaluation of EEG and QEEG literature on schizophrenia is complicated by the evident heterogeneity of the illness and the diversity of medication histories and dosage levels at the time of examination. In spite of these potential sources of difference among findings, considerable agreement nonetheless appears.
Across a large number of EEG and QEEG studies, there is a broad consensus that schizophrenia shows a high incidence of EEG and QEEG abnormalities. Most often, the reported abnormalities have been delta and/or theta excesses in frontal areas, a decreased mean frequency and lower power in the alpha band, and increased beta power. Increased anterior coherence also has often been reported. Coherence measures may contribute to distinguishing bipolar disorder from schizophrenia.
Mood Disorders: Unipolar and Bipolar Depression
The incidence of abnormal conventional EEG findings in mood disorders appears to be substantial, ranging from 20% to 40%2,97,146- 148 higher in 1) manic than depressed patients, 2) female than male bipolar patients, and 3) nonfamilial cases with late-age onset. Whether an "abnormal" EEG is a necessary correlate of a clinically effective series of ECT treatment is controversial. This suggestion, like that made above regarding clozapine in schizophrenia, will require further study.
Specific patterns noted in mood-disordered patients include the controversial small sharp spikes (SSS), 6/s spike and wave complexes, and positive spikes, seen especially in patients with suicidal ideation.2,149,150 The SSS pattern appears often in bipolar patients and also in their first-degree relatives.151
Numerous QEEG studies have found increased alpha and/or theta power in a high percentage of depressed patients.8,19,152- 157 Antidepressants reduce alpha activity,153,158- 161 suggesting normalization of these deviant QEEG features (in contrast to the increased alpha caused by neuroleptics).113- 115 Interhemispheric asymmetry, especially in anterior regions, has been reported repeatedly,162- 166 as has decreased coherence.19,121,167 In bipolar illness, in contrast to unipolar depression, alpha activity is reduced155,168 and beta activity increased.5,19 This difference may serve to separate unipolar from bipolar patients presenting in a state of depression without prior history of mania.5,167
Current treatment of bipolar disorder often includes the use of the anticonvulsant medications carbamazepine and sodium valproate. The successful use of these agents suggests overlap between convulsive disorders and bipolar illness. Ruling out convulsive illness with EEG studies prior to initiation of anticonvulsant treatment in bipolar patients may be prudent.
Conclusion:
Both EEG and QEEG studies report that a high proportion of patients with mood disorders display abnormal brain electrical activity. EEG studies report that small sharp spikes and paroxysmal events are often found, especially on the right hemisphere, and that abnormal sleep studies are common.
There is broad consensus in QEEG studies that increases in alpha or theta power, as well as asymmetry and hypocoherence in anterior regions, appear most often in unipolar depressed patients. Bipolar patients often display decreased alpha but increased beta activity.
Mood Disorders: Anxiety, Panic, Obsessive-Compulsive, and Eating Disorders
Several studies suggest a high incidence of EEG abnormalities in patients with anxiety disorders, panic disorders, and obsessive-compulsive disorder (OCD).2,169- 172 Diminished alpha activity has been found in anxiety disorder by using QEEG,173,174 and increased theta activity has been reported in OCD.175,176 Two subtypes of OCD patients have been described. One, with increased alpha relative power, responded positively (82%) to serotonergic antidepressants, while the second, with increased theta relative power, failed to improve (80%).177 Epileptiform activity can occasionally be found in patients with tics (or stuttering), in addition to nonspecific diffuse slow activity.178,179 In patients with panic disorder, paroxysmal activity was four times more common than in depressed patients.180 Temporal lobe abnormalities, in particular, have been emphasized in QEEG studies in this type of patient.
In anorexia nervosa, abnormal background activity in the EEG can be seen in nearly 60% of patients, possibly related to the effect of starvation on cerebral metabolism. Paroxysmal abnormalities are seen in about 12% of these patients.181 In intractable binge eating, "soft" neurological and EEG signs can appear. Both anticonvulsant and antidepressant drugs have been helpful in some of these patients.182- 184 Patients with eating disorders frequently give a history of physical or sexual abuse as children, so the increase in EEG abnormalities in this group may be related to their abuse history. Alternatively, dietary and nutritional deficiencies may contribute to altered brain function.
Conclusion:
Although abnormalities have been reported repeatedly in EEG and QEEG studies of patients in the above categories, consistent patterns have not yet been discerned.
Developmental Learning Disorders, Attention Deficit Disorders, and Autism
Specific developmental learning disorders (SDLD) are estimated to affect 4% to 6% of all school-age children.21,185 Attention deficit disorders with or without hyperactivity (ADHD or ADD) have a prevalence of 6% to 9% in school-age children.186,187 Although ADD/ADHD and SDLD are believed to be distinct neuropsychiatric entities, there is considerable comorbidity between the two disorders. Precise and accurate determination of the presence of ADD/ADHD versus SDLD can be of critical importance in avoiding the potentially devastating impact of these disorders on children and their families. EEG and QEEG can contribute usefully to this distinction as well as to separating children with social or motivational factors underlying school problems from those with organic dysfunction.
The conventional EEG has been reported to be abnormal in 30% to 60% of children with ADHD or with specific learning disability (SDLD or LD), as reviewed by several authors.2,16,186,188- 191 Reported abnormalities have often included diffuse slowing and decreased alpha activity.
In QEEG studies, a high incidence of excess theta or decreased alpha and/or beta activity has been reported in SDLD children,18,21,33,192,186,187,193- 197 with theta or alpha excess often seen in children with ADD or ADHD. The types of QEEG abnormality found in SDLD children are related to academic performance.198 A large percentage of children with attention deficit problems (more than 90%) show QEEG signs of cortical dysfunction, the majority displaying frontal theta or alpha excess, hypercoherence, and a high incidence of abnormal interhemispheric asymmetry.197,199 Using QEEG measures, it has been possible to discriminate replicably ADD/ADHD versus normal children, with a specificity of 88% and a sensitivity of 94%,200 and ADD versus SDLD children, with a sensitivity of 97% and a specificity of 84.2%.200
The EEG is frequently abnormal in autism. In 14 studies encompassing approximately 800 patients, the mean incidence of abnormal EEGs was 50% (median 47%), but the range of values for the incidence of abnormalities was considerable (10%- 83%). This large range undoubtedly arose from differences both in the populations under study and, more important, the criteria used for abnormality. EEG abnormality can help predict a poorer outcome with regard to intelligence, speech, and educational achievement.151 Although clinical seizures are uncommon in autism, epileptiform activity sometimes occurs.
Conclusion:
Numerous EEG as well as QEEG reports agree that a high proportion of children with developmental disorders—among which learning disabilities and attention-deficit hyperactivity have received the most attention—display abnormal brain electrical activity.
There is a wide consensus that delta or theta excess and alpha and beta deficits are commonly encountered in children with learning disorders and that theta or alpha excesses are often seen in children with ADD/ADHD.
Alcohol and Substance Abuse
The changes during acute alcoholic intoxication include the slowing of the EEG, seen in the form of decreased alpha frequency and abundance, an increased amount of theta, and even some generalized delta rhythms.201 These slow waves have a relationship with the degree of intoxication. The extent of the disturbance of consciousness is related to the amount of slow activity.202
For chronic alcoholism, as in the acute stage, an increase in slow activity is often seen. This change appears as a decrease in alpha frequency and abundance, related to the typical blood alcohol level of a given patient, and also an increase in the theta rhythm, especially on the temporal areas.203,204 Temporal and frontal areas may also display an increase in fast activity related to the neuropsychological impairment, which must be distinguished from muscle artifact and often characterizes these records.128,203 Family history of alcoholism plays a prominent role in the risk of the disease.204- 208 In the subacute encephalopathy associated with alcoholism, not only are slow waves noted, but epileptiform activity can also be seen, even as periodic lateralized epileptiform discharges (PLEDS).209
Recent studies of substance abuse have largely relied on QEEG. Replicated reports have appeared of an increased beta (relative power) in alcohol dependence.19,128,203,204,207 Increased alpha power, especially in anterior regions, has been reported in withdrawal, as well as after acute exposure to cannabis.210,211 Increased alpha and decreased delta and theta have been reported in crack cocaine users in withdrawal.212- 216
Conclusion:
There is a broad consensus that both EEG and QEEG reveal marked abnormalities in alcoholics and substance abusers. The effects vary depending on the drug. Either increased slow activity with lower alpha and beta or the converse have been reported; this reflects the diversity of substances or states focused upon.
However, among numerous QEEG studies, there is a consensus of increased beta relative power in alcoholism and increased alpha in cannabis or crack cocaine users.
Mild Head Injury or Postconcussion Syndrome
Patients with complaints of cognitive memory or attentional deficit after mild head injury without loss of consciousness frequently present for psychiatric evaluation for worker's compensation and disability benefits. Objective evidence of brain dysfunction in such cases is critical in the endeavor to separate the truly dysfunctional patient from the malingerer. EEG/QEEG evidence can play a critical role in such cases. Although the absence of abnormal brain electrical activity cannot definitively exclude the possibility of brain dysfunction, the presence of abnormalities, especially those most frequently associated with unequivocal traumatic brain insult, must be considered supportive of such claims.
There is a high incidence of diffuse axonal injury, about 50%, in the 50,000 patients per year with head injury who recover; these patients characteristically do not display structural lesions on CT or even on MRI scans early after injury.217,218 Among those who recover after moderate head injury, 90% have cognitive or neuropsychological deficits.219 Among such patients, studies involving many hundreds of cases have reported normal neurologic examinations but abnormal EEG.220- 226 Numerous EEG and QEEG studies of severe head injury (Glasgow Coma Scale [GCS] 4- 8) and moderate injury (GCS 9- 12), using samples of 50 to 200 patients, have agreed that increased theta and decreased alpha power and/or decreased coherence and asymmetry often characterize such patients. Changes in these measures provide the best predictors of long-term outcome.219,227- 229
The studies cited above generally concur that the most characteristic QEEG or EEG abnormalities persisting after mild or moderate head injury are similar in type to those found after severe head injury, namely increased power in the theta band, decreased alpha, low coherence, and increased asymmetry. It is noteworthy that similar EEG abnormalities have been reported in boxers,230 correlated with the numbers of bouts or knockouts, and in professional soccer players who were "headers."231 These observations lend further credibility to the multiple reports of discriminant functions based on QEEG variables that successfully separated normal individuals from patients with a history of mild to moderate head injury years after apparent clinical recovery.232,233
Conclusion:
The consistency of these EEG and QEEG findings is high across the range from mild to moderate head injury as well as in sports-related head impacts, and similar types of abnormalities are observed in patients with severe head injury as they recover.
There is a broad consensus that increased focal or diffuse theta, decreased alpha, decreased coherence, and increased asymmetry are common EEG indicators of the postconcussion syndrome.
Three-Dimensional Statistical Probability Images: Source Localization and QEEG Tomography
In the last decade, the problem of three-dimensional localization of the sources of voltage fields detected on the scalp has been energetically attacked by mathematicians and physicists as well as neurophysiologists. Basic early approaches have been reviewed by Lütkenhöner et al.234 and Buckner et al.235
The initial success in applying these new source localization techniques to electrophysiological signals was achieved by brain electrical source analysis, or BESA.236- 238 This method has received widespread acceptance for the separation and identification of highly localized dipole generators, such as focal epileptic discharges or spikes, and some neurosurgical centers have begun to use the method to pinpoint surgical targets in patients with intractable seizures.
Attention was directed next to localization of multiple sources, and then to the related problem of distributed sources, by the use of deblurring techniques.239,240 Approaches to the localization of distributed sources have been explored in simulations, in patients with space-occupying lesions, and in psychiatric patients. These approaches have included localization of the centroids of FFT power maps,241 frequency domain localization,234 linearly constrained minimum variance spatial filtering,242,243 recursive minimum norms,244 optimal resolution kernels and the resolution field,245,246 a Bayesian approach using anatomofunctional constraints,247 and combinatorial optimization applied to finite element models.235 Maximum likelihood procedures have been used to search for equivalent dipoles in 36 patients with space-occupying lesions. In 75% of these cases, the origin of deviant delta activity was localized near the center of the lesion volume, even in deep subcortical regions.248
Perhaps the most promising approach to the inverse solution of distributed sources is low-resolution electromagnetic tomography (LORETA),249 a generalized minimum norm technique that has been analytically compared with several different solutions to the inverse problem and shown to be optimal.250 It has been applied to the localization of intracranial epileptiform activity, using subdural electrodes to confirm inferences from scalp,251 and to localizing space-occupying lesions with known positions based on CT scans.248,252 Locations defined by LORETA were found to correspond well to the actual position of the abnormalities.
Using LORETA, a group of 9 never-medicated first-break acute schizophrenic patients was compared voxel by voxel with a group of 36 normal control subjects, indicating stronger activity in the right frontal regions but weaker activity in posterior regions in the schizophrenic patients than in the normal subjects.253 These findings were in agreement with reports of hyperfrontality in some schizophrenic patients.
Topographic mapping of quantitative electrophysiological data has recently been extended to quantitative electroencephalographic tomography, or QEEG-T, by Valdes-Sosa.254 A standard probabilistic brain atlas in Talairach space, constructed from more than 300 MRIs of normal individuals,255 was evaluated, voxel by voxel, to constrain the hypothetical generators of the distributed inverse solution to gray matter. Regression equations were then computed for the log spectra of sources in every voxel of gray matter (3,500 voxels). These voxel-normative data spanned the age range 5 to 97 years and were calculated over spectra ranging from 0.39 to 19 Hz, in 0.39 Hz increments. Statistical probability tomographic images (SPTI) were then obtained by Z-transformation of each voxel of the raw LORETA image obtained from an individual with respect to the corresponding voxel-normative data. Spatial statistical methods evaluated deviations from the norms of regions of interest, and results were displayed on slices from the probabilistic atlas with each voxel color coded for statistical significance.
QEEG-T has been used to seek CNS signs of HIV infection.256 Observed changes were of particular interest because of similarity to those found with increasing cognitive deterioration in aging. When LORETA was used to study 319 elderly patients staged by scores on the Global Deterioration Scale, systematic changes in certain brain regions were found with increasing cognitive impairment.257
Positive findings with different anatomical distributions were also found among five subtypes of schizophrenic patients identified by cluster analysis,138 OCD patients who were responders or nonresponders to selective serotonin reuptake inhibitors,77 and children with ADHD who were or were not clinical responders to stimulant therapy.200
Conclusion:
The combination of QEEG with the distributed inverse solution technology of LORETA, along with the projection of statistically evaluated voxel source values on a probabilistic MRI atlas, may extend the clinical relevance of QEEG, yield valuable insights into the pathophysiology underlying some psychiatric disorders, and provide clues useful for the rationalization of pharmacotherapy.

|
SUMMARY |
Both conventional EEG and QEEG studies provide valuable information to the psychiatrist regarding diagnosis and treatment responsiveness.
Conventional EEG is most useful in the following:
- Identifying paroxysmal activity.
- Identifying gross alterations in the background frequencies of the EEG.
- Identifying intermixed slow activity that may be related to delirium or dementia.
- Evaluating sleep disorders.
Conventional EEG assessments should be included in the diagnostic workup for the following:
- An acute confusional state.
- The first presentation of schizophrenia.
- A major mood disorder or mania.
- Refractory behavioral problems such as obsessions, violence, or panic.
Quantitative EEG studies are particularly well suited to identifying subtle changes in the topographic distribution of background activity. They can aid difficult differential diagnoses, such as:
- Distinguishing between delirium or dementia and depression.
- Distinguishing between schizophrenia and mood disorders.
- Assessing cognitive, attentional, or developmental disorders.
- Distinguishing between environmentally induced and endogenously mediated behavioral disorders.
- Evaluating alcohol or substance abuse.
- Evaluating postconcussion syndrome.
In dementia, multiple articles appearing within the last 5 years strongly suggest that QEEG may enable early detection and prognosis of future cognitive impairment. Such information might aid in development of prophylactic rather than remedial pharmacotherapeutic interventions, intended to prevent or slow further progress of the illness.
Some QEEG studies show promise of predicting the likelihood of response to a particular pharmacologic treatment and of monitoring responsiveness to treatment. The potential clinical value of QEEG procedures for analytic selection of the treatment most likely to be efficacious would be enormous in learning and attentional disorders and in schizophrenia.
Considerable attention is currently being given to the correlation of EEG or QEEG brain mapping with other brain functional mapping methods such as PET, SPECT, and MRI. Methods have been developed for the estimation of three-dimensional electrical source distributions in the brain computed from scalp recordings. If these source localization methods can be validated by more direct brain imaging using functional and structural MRI, PET, and SPECT, low-resolution electrical tomography offers the possibility of readily available, low-cost functional 3D brain images computed from QEEG recordings.
We have adopted the general procedures and the "quality of evidence" and the "strength of recommendation" ratings used in the Report to the American Academy of Neurology and the American Clinical Neurophysiology Society4 to evaluate conclusions drawn from the evidence cited above.
Procedures
The general procedures used in the special report required eight major categories to be considered in evaluating a procedure. Those issues are listed below and the body of relevant QEEG evidence summarized for each one:
- Disease studied is clearly defined. This criterion is definitively satisfied by the general use of DSM classification in the majority of recent studies cited herein.
- Criteria for test abnormality defined explicitly, clearly, and prospectively. The criteria for QEEG test abnormality are defined by parametric statistical methods and are satisfied in the majority of recent studies cited.
- Control groups not included in normative groups. Almost all of the recent studies cited compared patients with independent control groups.
- Same degree of severity in study as in probable use. Many of the studies cited used patients categorized as early onset or mildly impaired, compatible with probable clinical utilization of these methods.
- Test-retest reliability high. Numerous studies of large samples of normal or clinical groups attest to high test-retest reliability.
- Sensitivity, specificity, positive predictive value, negative predictive value demonstrated. Requirement of a replicated finding significant at the P<0.05 level provides an expected specificity of about 95%. Sensitivities range from 60% to 95% in the reports cited here, depending on the disorder.
- Validity compared with other tests for same difference diagnosis. No biological tests have been validated for the psychiatric disorders discussed herein. Although MRI and CT have high specificity, they are not definitively sensitive even in mild dementia or mild head injury.
- Medical efficacy tests reduce morbidity by clarifying best intervention, clarifying diagnosis, giving more accurate prognosis, and providing a less risky substitute for previous method. Present treatments of the conditions of major concern discussed herein are symptom-based, diagnoses are empirical, prognosis is probabilistic, and treatment is selected by trial and error. In view of the absence of existing methods that offer objective biological adjuncts to diagnosis, selection of treatment, or accurate predictive value, QEEG methods offer improved efficacy of patient management and decrease the risk of ineffectual treatment or misdiagnosis.
Quality of Evidence Ratings
Class I:
Evidence provided by one or more well-designed, prospective, blinded, controlled clinical studies.
Class II:
Evidence provided by one or more well-designed clinical studies, such as case control or cohort studies.
Class III:
Evidence provided by expert opinion, nonrandomized historical controls, or case reports of one or more.
Strength of Recommendation Ratings
Type A:
Strong positive recommendation, based on Class I evidence or overwhelming Class II evidence.
Type B:
Positive recommendation, based on Class II evidence.
Type C:
Positive recommendation, based on strong consensus of Class III evidence.
Type D:
Negative recommendation, based on inconclusive or conflicting Class II evidence.
Type E:
Negative recommendation, based on evidence of ineffectiveness or lack of efficacy.
Recommendations
The special article that presents the opinions of joint committees of the American Academy of Neurology and the American Clinical Neurophysiology Society4 concluded that QEEG might be a useful adjunct in the evaluation of patients with symptoms of cerebrovascular disease or dementia, making Type B recommendations (positive, based on Class II evidence provided by one or more well-designed clinical studies) for such use of QEEG. The special article further concluded that QEEG remains investigational for clinical use in postconcussion syndrome or mild head injury, learning disability, attention disorders, schizophrenia, depression, alcoholism, and drug abuse, with a Type D recommendation (negative, based on inconclusive or conflicting Class II evidence).
Basing our judgment on the prescribed procedures and adopting the rating scales used in that special article, we believe that the studies summarized in this review adequately justify the assessments of the utility of QEEG in the major categories of illness that follow:
Cerebrovascular Disease:
On the basis of many concordant Class II studies, Type B recommendation.
Dementia:
On the basis of multiple Class I and many concordant Class II studies, Type A recommendation.
Learning and Attention Disorders:
On the basis of multiple Class II studies and abundant Class II evidence, Type B recommendation.
Mood Disorders:
On the basis of multiple Class II studies, Type B recommendation.
Postconcussion Syndrome:
On the basis of several Class II studies and multiple concordant Class III studies, Type C recommendation.
Schizophrenia:
On the basis of conflicting Class II and III evidence, Type D recommendation.
Substance Abuse:
On the basis of conflicting Class II and III evidence, Type D recommendation.
Clinical Implications
In view of the accumulation of positive findings surveyed in this article, more psychiatrists may wish to explore the utility of these methods for themselves and begin to apply them in their clinical practice. EEG, first clinically applied in 1929 by the neuropsychiatrist Hans Berger,258 promises to have greatly expanded use as psychiatrists become more familiar with its many applications.

|
REFERENCES |
- American Electroencephalographic Society: Statement on clinical use of quantitative EEG. J Clin Neurophysiol 1987; 4:75
- Small JG: Psychiatric disorders and EEG, in Electroencephalography: Basic Principles, Clinical Applications, and Related Fields, edited by Niedermeyer E, Lopes da Silva F. Baltimore, Williams and Wilkins, 1993, pp 581- 596
- Hughes JR: The EEG in psychiatry: an outline with summarized points and references. Clin Electroencephalogr 1995; 26:92- 101
[Medline]
- Nuwer M: Assessment of digital EEG, quantitative EEG and EEG brain mapping: report of the American Academy of Neurology and the American Clinical Neurophysiology Society. Neurology 1997; 49:277- 292
[Abstract]
- Prichep LS, John ER: Neurometrics: Clinical applications, in Clinical Applications of Computer Analysis of EEG and Other Neurophysiological Variables, edited by Lopes da Silva FH, Storm van Leeuwen W, Remond A; vol 2 of Handbook of Electroencephalograpy and Clinical Neurophysiology. Amsterdam, Elsevier, 1986, pp 153- 170
- American Academy of Neurology: Assessment: EEG brain mapping. Neurology 1989; 13:1100- 1101
- American Psychiatric Association Task Force on Quantitative EEG Techniques: Quantitative electroencephalography: a report on the present state of computerized EEG techniques. Am J Psychiatry 1991; 148:961- 964
[Abstract]
- Alper K: Quantitative EEG and evoked potentials in adult psychiatry, in Advances in Biological Psychiatry, vol 1, edited by Panksepp J. Greenwich, CT, JAI Press, 1995, pp 65- 112
- Llinás RR: The intrinsic properties of mammalian neurons: insights into central nervous system function. Science 1988; 242:1654- 1664
- Lopes da Silva FH: The generation of electric and magnetic signals of the brain by local networks, in Comparative Human Physiology, edited by Greger R, Windhorst U. Berlin, Springer-Verlag, 1996, pp 509- 531
- McCormick DA: Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Prog Neurobiol 1992; 39:337- 388
[Medline]
- Steriade M, Gloor P, Llinás RR, et al: Basic mechanisms of cerebral rhythmic activities. Electroencephalogr Clin Neurophysiol 1990; 76:481- 508
[Medline]
- Matousék M, Volavka J, Roubícek J, et al: EEG frequency analysis related to age in normal adults. Electroencephalogr Clin Neurophysiol 1967; 23:162- 167
[Medline]
- Matousék M, Petersén I: Norms for the EEG, in Automation of Clinical Electroencephalography, edited by Kellaway P, Petersén I. New York, Raven, 1973, pp 75- 102
- John ER, Ahn H, Prichep LS, et al: Developmental equations for the electroencephalogram. Science 1980; 210:1255- 1258
- Fein G, Galin D, Johnstone J, et al: EEG power spectrum in normal and dyslexic children, I: reliability during passive conditions. Electroencephalogr Clin Neurophysiol 1983; 55:399- 405
[Medline]
- Oken BS, Chiappa KH: Short-term variability in EEG frequency analysis. Electroencephalogr Clin Neurophysiol 1988; 69:191- 198
[Medline]
- John ER, Prichep LS, Ahn H, et al: Neurometric evaluation of cognitive dysfunctions and neurological disorders in children. Prog Neurobiol 1983; 21:239- 290
[Medline]
- John ER, Prichep LS, Fridman J, et al: Neurometrics: computer assisted differential diagnosis of brain dysfunctions. Science 1988; 293:162- 169
- John ER, Prichep LS: Principles of neurometrics and neurometric analysis of EEG and evoked potentials, in EEG: Basic Principles, Clinical Applications, and Related Fields, edited by Niedermeyer E, Lopes da Silva F. Baltimore, Williams and Wilkins, 1993, pp 989- 1003
- Ahn H, Prichep LS, John ER, et al: Developmental equations reflect brain dysfunction. Science 1980; 210:1259- 1262
- Gasser T, Bacher P, Mochs J: Transformation towards the normal distribution of broad-band spectral parameters of the EEG. Electroencephalogr Clin Neurophysiol 1982; 53:119- 124
[Medline]
- Jonkman EJ, Poortvliet DCJ, Veering MM, et al: The use of neurometrics in the study of patients with cerebral ischemia. Electroencephalogr Clin Neurophysiol 1985; 61:333- 341
[Medline]
- Yingling CD, Galin D, Fein G, et al: Neurometrics does not detect "pure" dyslexics. Electroencephalogr Clin Neurophysiol 1986; 63:426- 430
[Medline]
- Harmony T, Alvarez A, Pascual R, et al: EEG maturation of children with different economic and psychosocial characteristics. Int J Neurosci 1987; 31:103- 113
- Alvarez A, Pascual R, Valdes P: U.S. EEG developmental equations confirmed for Cuban schoolchildren. Electroencephalogr Clin Neurophysiol 1987; 67:330- 332
[Medline]
- Diaz de Leon AE, Harmony T, Marosi E, et al: Effect of different factors on EEG spectral parameters. Int J Neurosci 1988; 43:123- 131
[Medline]
- Ritchlin CT, Chabot RJ, Alper K, et al: Quantitative electroencephalography: a new approach to the diagnosis of cerebral dysfunction in systemic lupus erythematosus. Arthritis Rheum 1992; 35:1330- 1342
- Valdes-Sosa P, Bosch J, Grave R, et al: Frequency domain models of the EEG. Brain Topogr 1992; 5:309- 319
- Veldhuizen RJ, Jonkman EJ, Poortvliet DCJ: Sex differences in age regression parameters of healthy adults-normative data. Electroencephalogr Clin Neurophysiol 1993; 86:377- 384
[Medline]
- Duffy FH, Albert MS, McAnulty GB: The pattern of age-related differences in electrophysiological activity of healthy subjects. Neurobiol Aging 1993; 14:73- 74
[Medline]
- Duffy FH, Jones K, McAnulty GB, et al: Spectral coherence in normal adults: unrestricted principal components analysis. Clin Electroencephalogr 1995; 26:30- 46
[Medline]
- Matsuura M, Okubo Y, Toru M, et al: A cross-national study of children with emotional and behavioral problems: a WHO collaborative study in the Western Pacific region. Biol Psychiatry 1993; 34:59- 65
[Medline]
- Swets JA: Measuring the accuracy of diagnostic systems. Science 1988; 240:1285- 1293
- Boutros NN: A review of indications of routine EEG in clinical psychiatry. Hospital and Community Psychiatry 1992; 43:716- 719
[Medline]
- Reilly EL, Glass G, Faillace LA: EEG's in an alcohol detoxification and treatment center. Clin Electroencephalogr 1979; 10:69- 71
[Medline]
- Kelley JT, Reilly EL: EEG, alcohol, and alcoholism, in EEG and Evoked Potentials in Psychiatry and Behavioral Neurology, edited by Hughes JR, Wilson WP. London, Butterworth, 1983, pp 55- 77
- Rosebush P, Stewart T: A prospective analysis of 24 episodes of neuroleptic malignant syndrome. Am J Psychiatry 1989; 146:717- 725
[Abstract]
- Jacobson SA, Leuchter AF, Walter DO: Conventional and quantitative EEG in the diagnosis of delirium among the elderly. J Neurol Neurosurg Psychiatry 1996; 56(suppl 2):153- 158
- Leuchter AF, Newton TF, Cook IA, et al: Changes in brain functional connectivity in Alzheimer-type and multi-infarct dementia. Brain 1992; 115:1543- 1561
- Robinson DJ, Merskey H, Blume WT, et al: Electroencephalography as an aid in the exclusion of Alzheimer's disease. Arch Neurol 1994; 51:280- 284
[Abstract]
- Harden CL, Daras M, Tuchman AJ, et al: Low amplitude EEGs in demented AIDS patients. Electroencephalogr Clin Neurophysiol 1993; 87:54- 56
[Medline]
- Fisch BJ, Pedley TA: The role of quantitative topographic mapping on "neurometrics" in the diagnosis of psychiatric and neurological disorders: the cons. Electroencephalogr Clin Neurophysiol 1989; 73:5- 9
[Medline]
- Brenner RP, Ulrich RF, Spiker DG, et al: Computerized EEG spectral analysis in elderly normal, demented and depressed subjects. Electroencephalogr Clin Neurophysiol 1986; 64:483- 492
[Medline]
- Hughes JR, Shanmugham S, Wetzel LC, et al: The relationship between EEG changes and cognitive functions in dementia: a study in a VA population. Clin Electroencephalogr 1989; 20:77- 85
[Medline]
- Duffy FH, Albert MS, McAnulty G: Brain electrical activity in patients with presenile and senile dementia of the Alzheimer type. Ann Neurol 1984; 16:439- 448
[Medline]
- Coburn KA, Danziger WL, Storandt M: A longitudinal EEG study of mild senile dementia of Alzheimer type: changes at 1 year and at 2.5 years. Electroencephalogr Clin Neurophysiol 1985; 61:101- 112
[Medline]
- Leuchter AF, Spar JD, Walter DO, et al: Electroencephalographic spectra and coherence in the diagnosis of Alzheimer's-type and multi-infarct dementia. Arch Gen Psychiatry 1987; 44:993- 998
[Abstract]
- Erkinjuntti T, Larsen T, Sulkava R, et al: EEG in the differential diagnosis between Alzheimer's disease and vascular dementia. Acta Neurol Scand 1988; 77:36- 43
[Medline]
- Schreiter-Gasser U, Gasser T, Ziegler P: Quantitative EEG analysis in early onset Alzheimer's disease: a controlled study. Electroencephalogr Clin Neurophysiol 1993; 86:15- 22
[Medline]
- Breslau J, Starr A, Sicotte N, et al: Topographic EEG changes with normal aging and SDAT. Electroencephalogr Clin Neurophysiol 1989; 72:281- 289
[Medline]
- Brenner RP, Reynolds CF, Ulrich RF: EEG findings in depressive pseudodementia and dementia with secondary depression. Electroencephalogr Clin Neurophysiol 1989; 72:298- 304
[Medline]
- Prinz PN, Vitiello MV: Dominant occipital (alpha) rhythm frequency in early stage Alzheimer's disease and depression. Electroencephalogr Clin Neurophysiol 1989; 73:427- 432
[Medline]
- Soininen H, Partanen J, Laulumaa V, et al: Longitudinal EEG spectral analysis in early stage of Alzheimer's disease. Electroencephalogr Clin Neurophysiol 1989; 72:290- 297
[Medline]
- Coburn KA, Danziger WL, Berg L: Replication of a study of frequency of the resting awake EEG in mild probable Alzheimer's disease. Electroencephalogr Clin Neurophysiol 1990; 75:148- 154
[Medline]
- Rice DM, Buchsbaum MS, Starr A, et al: Abnormal EEG slow activity in left temporal areas in senile dementia of the Alzheimer type. J Gerontol 1990; 45:145- 151
- Streletz LJ, Reyes PF, Zolewska M, et al: Computer analysis of EEG activity in dementia of the Alzheimer type and Huntington's disease. Neurobiol Aging 1990; 11:15- 20
[Medline]
- Williamson PC, Merskey H, Morrison S, et al: Quantitative electrophysiologic correlates of cognitive decline in normal elderly subjects. Arch Neurol 1990; 47:1185- 1188
- Heikala EL, Laulumaa V, Soikkeli R, et al: Slow wave activity in the spectral analysis of the electroencephalogram is associated with cortical dysfunction in patients with Alzheimer's disease. Behav Neurosci 1991; 105:409- 415
[Medline]
- Hier DB, Mangone CA, Ganellen R, et al: Quantitative measurement of delta activity in Alzheimer's disease. Clin Electroencephalogr 1991; 22:178- 182
[Medline]
- Saletu B, Anderer P, Paulus E, et al: EEG brain mapping in diagnostic and therapeutic assessment of dementia. Alzheimer Dis Assoc Disord 1991; 5(1, suppl):S57- S75
- Mody CK, McIntyre HB, Miller BL, et al: Computerized EEG frequency analysis and topographic brain mapping in Alzheimer's disease. Ann NY Acad Sci 1991; 620:45- 46
[Medline]
- Soininen H, Partanen J, Laulumaa V, et al: Serial EEG in Alzheimer's disease:3 year follow-up and clinical outcome. Electroencephalogr Clin Neurophysiol 1992; 79:342- 348
- Leuchter AF, Cook JA, Newton TF, et al: Regional differences in brain electrical activity in dementia: use of spectral power and spectral ratio measures. Electroencephalogr Clin Neurophysiol 1993; 87:385- 393
[Medline]
- Anderer P, Saletu B, Klöppel B, et al: Discrimination between patients and normals based on topographic EEG slow wave activity: comparison between Z statistics, discriminant analysis and artificial neural network classifiers. Electroencephalogr Clin Neurophysiol 1994; 91:108- 117
[Medline]
- Pritchard WS, Duke DW, Coburn KL, et al: EEG-based, neural-net predictive classification of Alzheimer's disease versus control subjects is augmented by non-linear EEG measures. Electroencephalogr Clin Neurophysiol 1994; 91:118- 130
[Medline]
- Sloan EP, Fenton GW, Kennedy NS, et al: Electroencephalography and single photon emission computed tomography in dementia: a comparative study. Psychol Med 1995; 25:631- 638
[Medline]
- Prichep LS, John ER, Reisberg B, et al: Quantitative EEG (QEEG) prediction of cognitive deterioration in normal subjectively impaired elderly: a longitudinal study. Psychiatry Res 1996
- Visser SL, Van Tilburg V, Hooijer C, et al: Visual evoked potentials (VEPs) in senile dementia (Alzheimer type) and in non-organic behavioural disorders in the elderly: comparison with EEG parameters. Electroencephalogr Clin Neurophysiol 1985; 60:115- 121
[Medline]
- Giannitrapani D, Collins J, Vassiliadis D: The EEG spectra of Alzheimer's disease. Int J Psychophysiol 1991; 10:259- 269
[Medline]
- Soininen H, Partanen VJ, Helkala EL, et al: EEG findings in senile dementia and normal aging. Acta Neurol Scand 1981; 65:59- 70
- Albert MS, Duffy FH, McAnulty GB: Electrophysiologic comparisons between two groups of patients with Alzheimer's disease. Arch Neurol 1990; 47:857- 863
[Abstract]
- Soininen H, Partanen J, Paakkonen A, et al: Changes in absolute power values of EEG spectra in the follow-up of Alzheimer's disease. Acta Neurol Scand 1991; 83:133- 136
[Medline]
- Rae-Grant A, Blume W, Lau C, et al: The electroencephalogram in Alzheimer-type dementia: a sequential study correlating the electroencephalogram with psychometric and quantitative pathologic data. Arch Neurol 1987; 44:50- 54
[Abstract]
- Dierks T, Perisic I, Frolich L, et al: Topography of the quantitative electroencephalogram in dementia of the Alzheimer type: relation to severity of dementia. Psychiatry Res 1991; 40:181- 194
[Medline]
- Richards M, Folstein M, Albert M, et al: Multicenter study of predictors of disease course in Alzheimer disease (the "predictors study"), II: neurological, psychiatric and demographic influences on baseline measures of disease severity. Alzheimer Dis Assoc Disord 1993; 7:22- 32
[Medline]
- Prichep LS, John ER, Ferris SH, et al: Quantitative EEG correlates of cognitive deterioration in the elderly. Neurobiol Aging 1994; 15:85- 90
[Medline]
- Hooijer C, Jonker C, Posthman J, et al: Reliability, validity and followup of the EEG in senile dementia: sequelae of sequential measurement. Electroencephalogr Clin Neurophysiol 1990; 76:400- 412
[Medline]
- Sloan EP, Fenton GW: EEG power spectra and cognitive change in geriatric psychiatry: a longitudinal study. Electroencephalogr Clin Neurophysiol 1993; 86:361- 367
[Medline]
- Rodriguez G, Nobili F, Arrigo A, et al: Prognostic significance of quantitative electroencephalography in Alzheimer patients: preliminary observations. Electroencephalogr Clin Neurophysiol 1996; 99:123- 128
[Medline]
- Berg L, Danziger WL, Storandt M, et al: Predictive features in mild dementia of Alzheimer's type. Neurology 1984; 34:563- 569
[Abstract]
- Ingvar DH, Sjolund B, Ardo A: Correlation between dominant EEG frequency, cerebral oxygen uptake and blood flow. Electroencephalogr Clin Neurophysiol 1976; 41:268- 276
[Medline]
- Sainio K, Stenberg D, Keskimaki I, et al: Visual and spectral EEG analysis in the evaluation of the outcome in patients with ischemic brain infarction. Electroencephalogr Clin Neurophysiol 1983; 56:117- 124
[Medline]
- Nuwer MR, Jordon SE, Ahn SS: Quantitative EEG is abnormal more often than routine EEG in mild stroke. Neurology 1987; 37:1153- 1159
- Nagata K: Topographic EEG in brain ischemia: correlation with blood flow and metabolism. Brain Topogr 1988; 1:97- 106
[Medline]
- Nagata K, Tagwa K, Hiroi S, et al: Electroencephalographic correlates of blood flow and oxygen metabolism provided by positron emission tomography in patients with cerebral infarction. Electroencephalogr Clin Neurophysiol 1989; 72:16- 30
[Medline]
- Jackel RA, Harner RN: Computed EEG topography in acute stroke. Neurophysiol Clin 1989; 19:185- 197
[Medline]
- Faught E: Current role of electroencephalography in cerebral ischemia. Stroke 1993; 24:609- 613
[Abstract]
- Stigsby B, Johannesson G, Ingvar DH: Regional EEG analysis and regional cerebral blood flow in Alzheimer's and Pick's diseases. Electroencephalogr Clin Neurophysiol 1981; 51:537- 547
[Medline]
- John ER, Prichep LS, Chabot RJ, et al: Monitoring brain function during cardiovascular surgery: hypoperfusion vs. microembolism as the major cause of neurological damage during cardiopulmonary bypass, in Heart and Brain, Brain and Heart, edited by Refsum H, Sulg I, Rasmussen K. Heidelberg, Springer-Verlag, 1989, pp 405- 421
- Passero S, Rocchi R, Vatli G, et al: Quantitative EEG mapping, regional cerebral blood flow and neuropsychological function in Alzheimer's disease. Dementia 1992; 6:148- 156
- Kwa VIH, Weinstein HC, Meyjes EFP, et al: Spectral analysis of the EEG and 99m-Tc-HMPAO SPECT scan in Alzheimer's disease. Biol Psychiatry 1993; 33:100- 107
[Medline]
- Martin-Loeches M, Gil P, Jimenez F, et al: Topographic maps of brain electrical activity in primary degenerative dementia of the Alzheimer type and multi-infarct dementia. Biol Psychiatry 1991; 29:211- 223
[Medline]
- Besthorn C, Forstl H, Geiger-Kabisch C, et al: EEG coherence in Alzheimer disease. Electroencephalogr Clin Neurophysiol 1994; 90:242- 245
[Medline]
- O'Connor KP, Shaw JC, Ongley CO: The EEG and differential diagnosis in psychogeriatrics. Br J Psychiatry 1979; 135:156- 162
[Abstract]
- Yener GG, Leuchter AF, Jenden D, et al: Quantitative EEG in frontotemporal dementia. Clin Electroencephalogr 1996; 27:61- 68
[Medline]
- Ellingson RJ: The incidence of EEG abnormality among patients with mental disorders of apparently nonorganic origin: a critical review. Am J Psychiatry 1954; 111:263- 275
[Free Full Text]
- Small JG, Milstein V, Sharpley PH, et al: Electroencephalographic findings in relation to diagnostic constructs in psychiatry. Biol Psychiatry 1984; 19:471- 487
[Medline]
- Shagass C: Twisted thoughts, twisted brain waves? In Psychopathology and Brain Dysfunction edited by Shagass C, Gershon S, Friedhoff AJ. New York, Raven, 1977, pp 353- 378
- Gattaz WF, Mayer S, Ziegler P, et al: Hypofrontality on topographic EEG in schizophrenia: correlations with neuropsychological and psychopathological parameters. Eur Arch Psychiatry Clin Neurosci 1992; 241:328- 332
[Medline]
- Primavera A, Fonti A, Novello P, et al: Epileptic seizures in patients with catatonic syndrome. J Neurol Neurosurg Psychiatry 1994; 57:1419- 1422
- Fenton GW, Fenwick PBC, Dollimore J, et al: EEG spectral analysis in schizophrenia. Br J Psychiatry 1980; 136:445- 455
[Abstract]
- Koukkou M: EEG states of the brain, information processing, and schizophrenic primary symptoms. Psychiatry Res 1982; 6:235- 244
[Medline]
- Stevens JR, Livermore A: Telemetered EEG in schizophrenia: Spectral analysis during abnormal behavior episodes. J Neurol Neurosurg Psychiatry 1982; 45:385- 395
[Abstract]
- Morihisa JM, Duffy FH, Wyatt RJ: Brain electrical activity mapping (BEAM) in schizophrenic patients. Arch Gen Psychiatry 1983; 40:719- 728
[Abstract]
- Dierks T, Maurer K, Ihl R, et al: Evaluation and interpretation of topographic EEG data in schizophrenic patients, in Topographic Brain Mapping of EEG and Evoked Potentials, edited by Maurer K. Berlin and Heidelberg, Springer-Verlag, 1989, pp 507- 517
- Merrin EL, Floyd TC: Negative symptoms and EEG alpha activity in schizophrenic patients. Schizophr Res 1992; 8:11- 20
[Medline]
- Itil TM: Qualitative and quantitative EEG findings in schizophrenia. Schizophr Bull 1977; 3:61- 80
[Medline]
- Colombo C, Gambini O, Macciardi F, et al: Alpha reactivity in schizophrenia and in schizophrenic spectrum disorders: demographic, clinical and hemispheric assessment. Int J Psychophysiol 1989; 7:47- 54
[Medline]
- Laurian S, Bryois C, Gaillard JM, et al: Some aspects of brain electrical activity in schizophrenia. Adv Biol Psychiatry 1984; 15:60- 68
- Kemali D, Maj M, Galderisi S, et al: Clinical, biological, and neuropsychological features associated with lateral ventricular enlargement in DSM-III schizophrenic disorder. Psychiatry Res 1986; 21:137- 149
- Karson CN, Coppola R, Daniel DG: Alpha frequency in schizophrenia: an association with enlarged cerebral ventricles. Am J Psychiatry 1988; 145:861- 864
[Abstract]
- Galderisi S, Maj M, Mucci A, et al: QEEG alpha 1 changes after a single dose of high-potency neuroleptics as a predictor of short-term response to treatment in schizophrenic patients. Biol Psychiatry 1994; 35:367- 374
[Medline]
- Saletu B, Kufferle B, Grunberger J, et al: Clinical, EEG mapping and psychometric studies in negative schizophrenia: comparative trials with amisulpride and fluphenazine. Neuropsychobiology 1994; 29:125- 135
[Medline]
- Schellenberg R, Milch W, Schwarz A, et al: Quantitative EEG and BPRS data following haldol-decanoate administration in schizophrenics. Int Clin Psychopharmacol 1994; 9:17- 24
- Niedermeyer E: EEG and clinical neurophysiology, in Electroencephalography: Basic Principles, Clinical Applications and Related Fields, edited by Niedermeyer E, Lopes da Silva F. Baltimore, Urban and Schwarzenberg, 1987, pp 97- 117
- Herrmann WM, Schaerer E: Pharmaco-EEG: computer EEG analysis to describe the projection of drug effects on a functional cerebral level in humans, in Handbook of Electroencephalography and Clinical Neurophysiology, revised, vol 2, edited by Lopes da Silva FH, Storm van Leeuwen W, Remond A. Amsterdam, Elsevier Science, 1986, pp 385- 445
- Kemali D, Vacca L, Marciano F, et al: Computerized EEG in schizophrenics. Neuropsychobiology 1980; 6:260- 267
[Medline]
- Etevenon P, Peron-Magnan P, Rioux P, et al: Schizophrenia assessed by computerized EEG. Adv Biol Psychiatry 1981; 6:29- 34
- Günther W, Breitline D: Predominant sensorimotor area left hemisphere dysfunction in schizophrenia measured by brain electrical activity mapping. Biol Psychiatry 1985; 20:515- 532
[Medline]
- Ford MR, Goethe JW, Dekker DK: EEG coherence and power in the discrimination of psychiatric disorders and medication effects. Biol Psychiatry 1986; 21:1175- 1188
- Muller HF, Achim A, Laur A, et al: Topography and possible physiological significance of EEG amplitude variability in psychosis. Acta Psychiatr Scand 1986; 73:665- 675
[Medline]
- Karson CN, Coppola R, Morihisa JM, et al: Computerized electroencephalographic activity mapping in schizophrenia. Arch Gen Psychiatry 1987; 44:514- 517
[Abstract]
- Williamson P, Mamelak M: Frontal spectral EEG findings in acutely ill schizophrenics. Biol Psychiatry 1987; 22:1021- 1024
- Galderisi S, Mucci A, Mignone ML, et al: QEEG mapping in drug-free schizophrenics: differences from healthy subjects and changes induced by haloperidol treatment. Schizophr Res 1992; 6:15- 24
- Westphal KP, Grozinger B, Diekmann V, et al: Slower theta activity over the midfrontal cortex in schizophrenic patients. Acta Psychiatr Scand 1990; 81:132- 138
[Medline]
- Matsuura M, Yoshino M, Ohta K, et al: Clinical significance of diffuse delta EEG activity in chronic schizophrenia. Clin Electroencephalogr 1994; 25:115- 121
[Medline]
- Coger RW, Dymond AM, Serafetinides EA: Electroencephalographic similarities between chronic alcoholics and chronic, nonparanoid schizophrenics. Arch Gen Psychiatry 1979; 36:91- 94
[Abstract]
- Lifshitz K, Lee KL, Susswein S: Long-term replicability of EEG spectra and auditory evoked potentials in schizophrenic and normal subjects. Neuropsychobiol 1987; 18:205- 211
- Omori M, Koshino Y, Murata T, et al: Quantitative EEG of elderly schizophrenic patients. Japanese Journal of Psychiatry and Neurology 1992; 46(suppl 3):681- 692
- Klein C, Andersen B, Thom E: Blinking, alpha-brain waves and smoking in schizophrenia. Acta Psychiatr Scand 1983; 87(suppl 3):172- 178
- Feinberg I, Braun M, Koresko RL, et al: Stage 4 sleep in schizophrenia. Arch Gen Psychiatry 1969; 21:262- 266
[Medline]
- Caldwell DF: Differential levels of stage IV sleep in a group of clinically similar chronic schizophrenic patients. Biol Psychiatry 1969; 1:131- 141
[Medline]
- Kupfer DJ, Reynolds CF: Electroencephalographic sleep changes in psychiatric patients, in EEG and Evoked Potentials in Psychiatry and Behavioral Neurology, edited by Hughes JR, Wilson P. Boston and London, Butterworth, 1983
- Tandon R, Shipley JE, Taylor S, et al: Electroencephalographic sleep abnormalities in schizophrenia: relationship to positive negative symptoms and prior neuroleptic treatment. Arch Gen Psychiatry 1992; 49:185- 194
[Abstract]
- Lifshitz K, Gradijan J: Spectral evaluation of the electroencephalogram: power and variability in chronic schizophrenics and control subjects. Psychophysiology 1974; 11:479- 490
[Medline]
- Shaw JC, Brooks S, Colter N, et al: A comparison of schizophrenic and neurotic patients using EEG power and coherence spectra, in Hemisphere Asymmetries of Function in Psychopathology, edited by Gruzelier J, Flor-Henry P. Amsterdam, Elsevier, 1979, pp 257- 284
- John ER, Prichep LS, Alper KR, et al: Quantitative electrophysiological characteristics and subtyping of schizophrenia. Biol Psychiatry 1994; 36:801- 826
[Medline]
- Czobor P, Volavka J: Pretreatment EEG predicts short-term response to haloperidol treatment. Biol Psychiatry 1991; 30:927- 942
[Medline]
- Czobor P, Volavka J: Quantitative EEG electroencephalogram effect of risperidone in schizophrenic patients. J Clin Psychopharmacol 1993; 13:332- 342
[Medline]
- Günther W, Steinberg R, Petsch R, et al: EEG mapping in psychiatry: Studies on type I/II schizophrenia using motor activation, in Topographic Brain Mapping of EEG and Evoked Potentials, edited by Maurer K. Berlin and Heidelberg, Springer-Verlag, 1989, pp 439- 450
- Shagass C, Roemer R: Evoked potential topography in unmedicated and medicated schizophrenics. Int J Psychophysiol 1991; 10:213- 224
[Medline]
- Merrin EL, Floyd TC, Fein G: EEG coherence in unmedicated schizophrenic patients. Biol Psychiatry 1989; 25:60- 66
[Medline]
- Pockberger H, Thau K, Lovrek A, et al: Coherence mapping reveals differences in the EEG between psychiatric patients and healthy persons, in Topographic Brain Mapping of EEG and Evoked Potentials, edited by Maurer K. Berlin and Heidelberg, Springer-Verlag, 1989, pp 451- 457
- Nagase Y, Okubo Y, Matsuura M, et al: EEG coherence in unmedicated schizophrenic patients: topographical study. Biol Psychiatry 1992; 32:1028- 1034
- Taylor MA, Abrams R: Prediction of treatment response in mania. Arch Gen Psychiatry 1981; 38:800- 803
[Abstract]
- Cook BL, Shukla S, Hoff AL: EEG abnormalities in bipolar affective disorder. J Affect Disord 1986; 11:147- 149
[Medline]
- McElroy SL, Keck PE Jr, Pope HG Jr, et al: Valproate in the treatment of rapid-cycling bipolar disorder. J Clin Psychopharmacol 1988; 8:275- 279
[Medline]
- Gibbs FA, Gibbs EL: Atlas of Electroencephalography. Reading, MA, Addison-Wesley, 1964
- Struve FA, Saraf KR, Arko RS, et al: Relationship between paroxysmal electroencephalographic dysrhythmia and suicide ideation and attempts in psychiatric patients, in Psychopathology and Brain Dysfunction, edited by Shagass C, Gershon S, Friedhoff AJ. New York, Raven, 1977, pp 199- 221
- Small JH, Small IF, Milstein V, et al: Familial associations with EEG variants in manic-depressive disease. Arch Gen Psychiatry 1975; 32:43- 48
[Abstract]
- Monakhov K, Perris C: Neurophysiological correlates of depressive symptomatology. Neuropsychobiol 1980; 6:268- 279
- Itil TM: The discovery of antidepressant drugs by computer-analyzed human cerebral bio-electrical potentials (CEEG). Prog Neurobiol 1983; 20:185- 249
[Medline]
- Nystrom C, Matousek M, Hallstrom T: Relationships between EEG and clinical characteristics in major depressive disorder. Acta Psychiatr Scand 1986; 73:390- 394
[Medline]
- Knott VJ, Lapierre YD: Computerized EEG correlates of depression and antidepressant treatment. Prog Neuropsychopharmacol Biol Psychiatry 1987; 11:213- 221
[Medline]
- Pollock VE, Schneider LS: Quantitative, waking EEG research on depression. Biol Psychiatry 1990; 27:757- 780
[Medline]
- Nieber D, Schlegel S: Relationships between psychomotor retardation and EEG power spectrum in major depression. Biol Psychiatry 1992; 25:20- 23
- Saletu B, Grunberger J: Classification and determination of cerebral bioavailability of fluoxetine: pharmaco-EEG and psychometric analyses. Clin Psychiatry 1985; 46(3):45- 52
- Itil TM, Itil KZ, Mukherjee S, et al: A dose-finding study with sertraline, a new 5-HT reuptake blocking antidepressant using quantitative pharmaco-EEG and dynamic brain mapping. Integrative Psychiatry 1989; 7:29- 38
- McClelland GR, Raptopoulos P, Jackson D: The effect of paroxetine on the quantitative EEG. Acta Psychiatr Scand 1989; 350(suppl):50- 52
- Saletu B, Grunberger J, Anderer P, et al: Pharmacodynamics of venlafaxine evaluated by EEG brain mapping, psychometry and psychophysiology. Br J Clin Pharmacol 1992; 33:589- 601
[Medline]
- Schaffer CE, Davidson RH, Saron C: Frontal and parietal electroencephalogram asymmetry in depressed and nondepressed subjects. Biol Psychiatry 1982; 18:753- 762
- Kemali D, Vacca L, Marciano F, et al: CEEG findings in schizophrenics, depressives, obsessives, heroin addicts and normals. Adv Biol Psychiatry 1981; 6:17- 28
- Henriques JB, Davidson RJ: Regional brain electrical asymmetries discriminate between previously depressed and healthy control subjects. Journal of Abnormal Psychology 1990; 99:22- 31
[Medline]
- Henriques JB, Davidson RJ: Left frontal hypoactivation in depression. Journal of Abnormal Psychology 1991; 100:535- 545
[Medline]
- Allen JJ, Iacono WG, Depue RA, et al: Regional electroencephalographic asymmetries in bipolar seasonal affective disorder before and after exposure to bright light. Biol Psychiatry 1993; 33:642- 646
[Medline]
- Prichep LS, John ER, Essig-Peppard T, et al: Neurometric subtyping of depressive disorders, in Plasticity and Morphology of the CNS, edited by Cazzullo CL, Invernizzi G, Sacchetti E, et al. London, M.T.P. Press, 1990
- Clementz BA, Sponheim SR, Iacono WG, et al: Resting EEG in first-episode schizophrenia patients, bipolar psychotic patients, and their first-degree relatives. Psychophysiology 1994; 31:486- 494
[Medline]
- Jenike MA, Brotman AW: The EEG in obsessive-compulsive disorder. J Clin Psychiatry 1984; 45:122- 124
[Medline]
- Abraham HD: Stimulants, panic, and BEAM EEG abnormalities. Am J Psychiatry 1989; 146:947- 948
- Louie AK, Lannon RA, Ketter TA: Treatment of cocaine-induced panic disorder. Am J Psychiatry 1989; 146:40- 44
[Abstract]
- Abraham HD, Duffy F: Computed EEG abnormalities in panic disorder with and without drug abuse. Biol Psychiatry 1991; 29:687- 690
[Medline]
- Buchsbaum MS, Hazlett E, Sicotte N, et al: Topographic EEG changes with benzodiazepine administration in generalized anxiety disorder. Biol Psychiatry 1985; 20:832- 842
[Medline]
- Siciliani O, Schiavon M, Tansella M: Anxiety and EEG alpha activity in neurotic patients. Acta Psychiatr Scand 1975; 52:116- 131
[Medline]
- Perros P, Young ES, Ritson JJ, et al: Power spectral EEG analysis and EEG variability in obsessive-compulsive disorder. Brain Topography 1992; 4:187- 192
[Medline]
- Silverman JS, Loychik SG: Brain-mapping abnormalities in a family with three obsessive-compulsive children. J Neuropsychiatry Clin Neurosci 1990; 2:319- 322
[Abstract]
- Prichep LS, Mas F, Hollander E, et al: Quantitative electroencephalographic (QEEG) subtyping of obsessive compulsive disorder. Psychiatry Res 1993; 50:25- 32
[Medline]
- Okasha A, Moneim AS, Bishry Z, et al: Electroencephalographic study of stammering. Br J Psychiatry 1974; 124:534- 535
[Medline]
- Gilroy J, Meyer JS: Medical Neurology. New York, Macmillan, 1975
- Jabourian AP, Erlich M, Desvignes C, et al: Panic-attacks and 24 hours EEG by out-patients. Annales Medico Psychologiques 1992; 150:240- 245
[Medline]
- Crisp AH, Fenton G, Sutton L: A controlled study of the EEG in anorexia nervosa. Br J Psychiatry 1968; 114:1149- 1169
- Rau JH, Green RS: Soft neurological correlates of compulsive eaters. J Nerv Ment Dis 1978; 166:435- 437
[Medline]
- Rau JH, Struve FA, Green RS: Electroencephalographic correlates of compulsive eating. Clin Electroencephalogr 1979; 10:180- 189
[Medline]
- Grebb JA, Yingling CD, Reus VI: Electrophysiologic abnormalities in patients with eating disorders. Compr Psychiatry 1984; 25:216- 223
[Medline]
- Krupp LB, Masur D, Schwartz J, et al: Cognitive functioning in late Lyme borreliosis. Arch Neurol 1991; 48:1125- 1129
- John ER: Functional Neuroscience, vol 2: Neurometrics: Clinical Applications of Quantitative Electrophysiology. Hillsdale, NJ, Lawrence Erlbaum, 1977
- Kaye H, John ER, Ahn H, et al: Neurometric evaluation of learning disabled children. Int J Neurosci 1981; 13:15- 25
[Medline]
- Fein G, Galin D, Yingling CD, et al: EEG spectra in dyslexic and control boys during resting conditions. Electroencephalogr Clin Neurophysiol 1986; 63:87- 97
[Medline]
- Brying RF, Salmi TK, Sainio KO, et al: EEG in children with spelling disabilities. Electroencephalogr Clin Neurophysiol 1991; 79:247- 255
[Medline]
- Flynn JM, Deering WM: Subtypes of dyslexia: investigation of Border's system using quantitative neurophysiology. Dev Med Child Neurol 1989; 31:215- 223
[Medline]
- Hughes JR: Electroencephalography and learning disabilities, in Progress in Learning Disabilities, edited by Myklebust HR. New York, Grune and Stratton, 1971, pp 18- 55
- John ER, Karmel BZ, Corning WC, et al: Neurometrics: numerical taxonomy identifies different profiles of brain functions within groups of behaviorally similar people. Science 1977; 196:1383- 1410
- Dykman RA, Holcomb PJ, Oglesby DM, et al: Electrocortical frequencies in hyperactive, learning-disabled, mixed, and normal children. Biol Psychiatry 1982; 17:675- 685
[Medline]
- Gasser T, Mochs J, Lenard HG, et al: The EEG of mildly retarded children: developmental, classificatory and topographic aspects. Electroencephalogr Clin Neurophysiol 1983; 55:131- 144
[Medline]
- Lubar JF, Bianchini KJ, Calhoun WH, et al: Spectral analysis of EEG differences between children with and without learning disabilities. Journal of Learning Disabilities 1985; 18:403- 408
[Medline]
- Lubar JF: Discourse on the development of EEG diagnostics and biofeedback for attention-deficit/hyperactivity disorders. Biofeedback and Self-Regulation 1991; 16:201- 224
[Medline]
- Marosi E, Harmony T, Sanchez L, et al: Maturation of the coherence of EEG activity in normal and learning-disabled children. Electroencephalogr Clin Neurophysiol 1992; 83:350- 357
[Medline]
- Harmony T, Hinojosa G, Marosi E, et al: Correlation between EEG spectral parameters and an educational evaluation. Int J Neurosci 1990; 54:147- 155
[Medline]
- Mann CA, Lubar J, Zimmerman A, et al: Quantitative analysis of EEG in boys with attention deficit hyperactivity disorder: controlled study with clinical implications. Pediatr Neurol 1992; 8:30- 36
[Medline]
- Chabot RJ, Serfontein G: Quantitative EEG profiles of children with attention deficit disorder. Biol Psychiatry 1996; 40:951- 963
[Medline]
- Begleiter H, Platz A: The effects of alcohol on the central nervous system in humans, in The Biology of Alcoholism, vol 2, edited by Kissen B, Begleiter B. New York, Plenum, 1972
- Engel GL, Rosenbaum M: Delirium, III: electroencephalographic changes associated with alcoholic intoxication. Arch Neurol Psychiatry 1945; 53:44- 50
- Coger RW, Dymond AM, Serafentinides EA, et al: EEG signs of brain impairment in alcoholism. Biol Psychiatry 1978; 13:729- 739
[Medline]
- Bauer LO, Hesselbrock VM: EEG autonomic and subjective correlates of the risk for alcoholism. J Stud Alcohol 1993; 54:577- 589
[Medline]
- Michael A, Mirza KAH, Mukundan CR, et al: Interhemispheric electroencephalographic coherence as a biological marker in alcoholism. Acta Psychiatr Scand 1993; 87:213- 217
[Medline]
- Ehlers CL, Schuckit MA: Evaluation of EEG alpha activity in sons of alcoholics. Neuropsychopharmacology 1991; 4:199- 205
[Medline]
- Gabrielli WF, Mednick SA, Volavka J, et al: EEGs in children of alcoholic fathers. Psychophysiology 1982; 19:404- 407
[Medline]
- Pollock VE, Volavka J, Goodwin DW, et al: The EEG after alcohol administration in men at risk for alcoholism. Arch Gen Psychiatry 1983; 40:857- 861
[Abstract]
- Niedermeyer E, Freund G, Krumholz A: Subacute encephalopathy with seizures in alcoholics: a clinical electroencephalographic study. Clin Electroencephalogr 1981; 12(suppl 3):113- 129
- Struve FA, Straumanis JJ, Patrick G, et al: Topographic mapping of quantitative EEG variables in chronic heavy marijuana users: empirical findings with psychiatric patients. Clin Electroencephalogr 1989; 20:6- 23
[Medline]
- Struve FA, Straumanis JJ, Patrick G: Persistent topographic quantitative EEG sequelae of chronic marijuana use: a replication study and initial discriminant function analysis. Clin Electroencephalogr 1994; 25:63- 75
[Medline]
- Alper KR, Chabot RJ, Kim AH, et al: Quantitative EEG correlates of crack cocaine dependence. Psychiatry Res 1990; 35:95- 106
[Medline]
- Alper KR, Chabot R, Prichep LS, et al: Crack cocaine dependence: discrimination from major depression using QEEG variables, in Imaging of the Brain in Psychiatry and Related Fields, edited by Maurer K. Berlin, Springer-Verlag, 1993, pp 289- 293
- Cornwell A, Roemer RA, Jackson P, et al: Paroxysmal-like EEG abnormalities associated with chronic polydrug abuse. Biol Psychiatry 1994; 35:692- 693
- Prichep LS, Alper KR, Kowalik SC, et al: Quantitative electroencephalographic characteristics of crack cocaine dependence. Biol Psychiatry 1996; 40:986- 993
[Medline]
- Roemer RA, Cornwell A, Jackson P, et al: Quantitative EEG measures: correlations with lifetime exposure to cocaine, alcohol, and marijuana in chronic polydrug abusers. Biol Psychiatry 1994; 35:624- 625
- Chesnut RM, Pitts, LH: Medical management of intracranial pressure, in Head Injury. Baltimore, Butterworth, 1993, pp 225- 246
- Gennarelli TA: Cerebral concussion and diffuse brain injuries, in Head Injury, 3rd edition, edited by Cooper PR. Baltimore, Williams and Wilkins, 1993, pp 137- 157
- Rimel RW, Giordani B, Barth JT, et al: Moderate head injury: completing the clinical spectrum of brain trauma. Neurosurgery 1982; 11:344- 351
[Medline]
- Tönnis A: Beobachtungen an frischen gedekten hirnschadigungen [Observations on recent brain injuries], in Das Hirntrauma, edited by Rehwald E. Stuttgart, Thieme, 1956, p 772
- Denny-Brown D: Brain trauma and concussion. Arch Neurol 1961; 5:1- 3
- Verjaal A: Trauma capitis, in Neurologie vor de algemene praktijk, edited by van den Bergh R, Folkerts JF. Brussels, Agon-Elsevier, 1972, pp 179- 187
- Courjon J, Scherzer S: Traumatic disorders, in Handbook of Electroencephalography and Clinical Neurophysiology, vol 14, edited by Remond A, Magnus O, Courjon J. Amsterdam, Elsevier, 1972, pp 8- 95
- Koufen H, Dichgans J: Háufigkeit und ablauf von traumatischen EEG-Veránderungenund ihre klinischen korrelationen [Clinical correlations of EEG changes in trauma and their evolution]. Fortschr Neurol Psychiatr 1978; 46:165- 177
- Jerret SA, Corsak J: Clinical utility of topographic EEG brain mapping. Clin Electroencephalogr 1988; 19:134- 143
[Medline]
- Hooshmand H, Beckner E, Radfor F: Technical and clinical aspects of topographic brain mapping. Clin Electroencephalogr 1989; 20:235- 247
[Medline]
- Bricolo A, Turazzi S, Faccioli F, et al: Clinical application of compressed spectral array in long-term EEG monitoring of comatose patients. Electroencephalogr Clin Neurophysiol 1978; 45:211- 225
[Medline]
- Steudel WI, Krüger J: Using the spectral analysis of the EEG for prognosis of severe brain injuries in the first post-traumatic week. Acta Neurochir (Wien) 1979; 28(suppl):40- 42
- Thatcher RW, Cantor DS, McAlaster R, et al: Comprehensive predictions of outcome in closed head-injured patients. Ann NY Acad Sci 1983, 82- 101
- Ross RJ, Cole M, Thompson JS, et al: Boxers: computer tomography, EEG, and neurological evaluation. JAMA 1983; 249:211- 213
[Abstract]
- Tysvaer AT, Stroll OV, Bachen I: Soccer injuries to the brain: a neurologic and electroencephalographic study of former players. Acta Neurol Scand 1989; 80:151- 156
[Medline]
- Randolph C, Miller MH: EEG and cognitive performance following closed head injury. Neuropsychobiology 1988; 20:43- 50
[Medline]
- Thatcher RW, Walker RA, Gerson I, et al: EEG discriminant analyses of mild head trauma. Electroencephalogr Clin Neurophysiol 1989; 73:94- 106
[Medline]
- Lütkenhöner B: Frequency domain localization of intracerebral dipolar sources. Electroencephalogr Clin Neurophysiol 1992; 82:112- 118
[Medline]
- Buckner H, Knoll G, Fuchs M, et al: Inverse localization of electric dipole current sources in finite element models of the human head. Electroencephalogr Clin Neurophysiol 1997; 102:267- 278
[Medline]
- Scherg M, Von Crainon D: Two bilateral sources of the late AEP as identified by a spatio-temporal dipole model. Electroencephalogr Clin Neurophysiol 1985; 62:32- 44
[Medline]
- Scherg M, Picton TW: Separation and identification of event-related potential components by brain electric source analysis. Electroencephalogr Clin Neurophysiol 1991; 87(suppl 42):24- 37
- Scherg M: Functional imaging and localization of electromagnetic brain activity. Brain Topogr 1992; 5:103- 111
[Medline]
- Gevins A, Le J, Martin NK, et al: High resolution EEG:124 channel recording, spatial deblurring and MRI integration methods. Electroencephalogr Clin Neurophysiol 1994; 90:337- 358
- Babiloni F, Babiloni C, Carducci F, et al: High resolution EEG: a new model dependent spatial deblurring method using a realistically shaped MR-constructed subject's head model. Electroencephalogr Clin Neurophysiol 1997; 102:69- 80
[Medline]
- Lehmann D, Michel CM: Intracerebral source localization for FFT power maps. Electroencephalogr Clin Neurophysiol 1990; 76:271- 276
[Medline]
- Van Veen BD, Joseph J, Hecox K: Localization of intracerebral sources of electrical activity via linearly constrained minimum variance spatial filtering. IEEE Workshop on Statistical Signal and Array Processing, 1992, pp 526- 529
- van Drongelen W, Yutchman M, Van Veen BD, et al: A spatial filtering technique to detect the localize multiple sources in the brain. Brain Topogr 1996; 939- 49
- Gorodnitsky IF, George JS, Ras BD: Neuromagnetic source imaging with FOCUSS: a recursive minimum norm algorithm. Electroencephalogr Clin Neurophysiol 1995; 95:231- 251
[Medline]
- Grave de Peralta Menendez R, Hauk O, Audino SG, et al: Linear inverse solutions with optimal resolution kernels applied to electromagnetic tomography. Human Brain Mapping 1997; 5:454- 467
- Lütkenhöner B, Grave de Peralta Menendez R: Theoretical model for case of concurrent or diffusely distributed sources in EEG/MEG. Electroencephalogr Clin Neurophysiol 1997; 102:326- 334
[Medline]
- Baillet S, Garmero L: A Bayesian approach to introducing anatomo-functional priors in the EEG/MEG inverse problem. IEEE Tran Biomed Eng 1997; 44:374- 385
- Fernandez-Bouzas A, Casanova R, Harmony T, et al: EEG frequency domain distributed inverse solutions in brain lesions (abstract). Electroencephalogr Clin Neurophysiol 1997; 103:195
- Pascual-Marqui RD, Michel CM, Lehmann D: Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain. Int J Psychophysiol 1994; 18:49- 65
[Medline]
- Pascual-Marqui RD: Low resolution brain electromagnetic tomography (LORETA) (abstract). Electroencephalogr Clin Neurophysiol 1997; 103:36
- Lantz G, Michel CM, Pascual-Marqui RD, et al: Extracranial localization of intracranial interictal epileptiform activity using LORETA (low resolution electromagnetic tomography). Electroencephalogr Clin Neurophysiol 1997; 102:414- 422
[Medline]
- Harmony T, Fernandez-Bouzas A, Marosi E, et al: Frequency source analysis in patients with brain lesions. Brain Topogr 1995; 8:109- 117
[Medline]
- Pascual-Marqui RD, Koukkon M, Lehmann D: Hyperfrontality in never-treated first-break schizophrenics demonstrated by low resolution electromagnetic tomography (LORETA) (abstract). Electroencephalogr Clin Neurophysiol 1997; 103:92
- Valdes-Sosa P: Quantitative electroencephalographic tomography (abstract). Electroencephalogr Clin Neurophysiol 1997; 103:19
- Evans AC, Collins DL, Mills SR, et al:3D statistical neuroanatomical models from 305 MRI volumes. Proceedings of IEEE-Nuclear Science Symposium and Medical Imaging Conference, 1993, pp 1813- 1817
- Valdes-Sosa P, Amador F, Virues T, et al: qEEG signs of HIV infection (abstract). Abstracts of the 14th International Congress of EEG and Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol 1997; 103:28
- John ER, Prichep LS, Valdes-Sosa P: Neurometric EEG classification and subtyping of psychiatric patients (abstract). Electroencephalogr Clin Neurophysiol 1997; 103:36
- Berger HA: On the electroencephalogram of man. Archiven des Psychiatrie und nerven Krankenheis 1937; 106:577- 584
This article has been cited by other articles: (Search Google Scholar for Other Citing Articles)
| |
 |

|
 |
K. L. Coburn, E. C. Lauterbach, N. N. Boutros, K. J. Black, D. B. Arciniegas, and C. E. Coffey
The Value of Quantitative Electroencephalography in Clinical Psychiatry:
A Report by the Committee on Research of the American
Neuropsychiatric Association
J Neuropsychiatry Clin Neurosci, November 1, 2006; 18(4): 460 - 500.
[Abstract] [Full Text] [PDF]
 |
 |
| |
| |
 |

|
 |
N. Boutros, L. Fraenkel, and A. Feingold
A Four-Step Approach for Developing Diagnostic Tests in Psychiatry:
EEG in ADHD as a Test Case
J Neuropsychiatry Clin Neurosci, November 1, 2005; 17(4): 455 - 464.
[Abstract] [Full Text] [PDF]
 |
 |
| |
|
 |

|
 |
N. Boutros, H. A. Mirolo, and F. Struve
Normative Data for the Unquantified EEG: Examination of Adequacy for
Neuropsychiatric Research
J Neuropsychiatry Clin Neurosci, February 1, 2005; 17(1): 84 - 90.
[Abstract] [Full Text] [PDF]
 |
 |
|
|
 |

|
 |

P. J. Marshall, N. A. Fox, and the BEIP Core Group
A Comparison of the Electroencephalogram between Institutionalized
and Community Children in Romania
J. Cogn. Neurosci., September 1, 2004; 16(8): 1327 - 1338.
[Abstract] [Full Text] [PDF]
 |
 |
|
| |
 |

|
 |

L. D. Gugino, L. S. Aglio, and A. Yli-Hankala
Monitoring the Electroencephalogram During Bypass Procedures
Seminars in Cardiothoracic and Vascular Anesthesia, June 1, 2004; 8(2): 61 - 83.
[Abstract] [PDF]
 |
 |
| |
|
 |

|
 |
K. N. Loganovsky and K. L. Yuryev
EEG Patterns in Persons Exposed to Ionizing Radiation as a Result of
the Chernobyl Accident. Part 2: Quantitative EEG Analysis in Patients
Who Had Acute Radiation Sickness
J Neuropsychiatry Clin Neurosci, February 1, 2004; 16(1): 70 - 82.
[Abstract] [Full Text] [PDF]
 |
 |
|
|
 |

|
 |

J. Stone and G. Moran
The utility of EEG in psychiatry and aggression
Psychiatr. Bull., May 1, 2003; 27(5): 171 - 172.
[Abstract] [Full Text] [PDF]
 |
 |
|
|
 |

|
 |
D. Fujii and I. Ahmed
Characteristics of Psychotic Disorder Due to Traumatic Brain Injury:
An Analysis of Case Studies in the Literature
J Neuropsychiatry Clin Neurosci, May 1, 2002; 14(2): 130 - 140.
[Abstract] [Full Text] [PDF]
 |
 |
|
|
 |

|
 |

P. O. Gunawardane, P. A. Murphy, and J. W. Sleigh
Bispectral index monitoring during electroconvulsive therapy under
propofol anaesthesia
Br. J. Anaesth., February 1, 2002; 88(2): 184 - 187.
[Abstract] [Full Text] [PDF]
 |
 |
|
|
 |

|
 |
K. N. Loganovsky and K. L. Yuryev
EEG Patterns in Persons Exposed to Ionizing Radiation as a Result of the
Chernobyl Accident: Part 1: Conventional EEG Analysis
J Neuropsychiatry Clin Neurosci, November 1, 2001; 13(4): 441 - 458.
[Abstract] [Full Text] [PDF]
 |
 |
|
|
 |

|
 |

L. D. Gugino, R. J. Chabot, L. S. Prichep, E. R. John, V. Formanek, and L. S. Aglio
Quantitative EEG changes associated with loss and return of consciousness
in healthy adult volunteers anaesthetized with propofol or sevoflurane
Br. J. Anaesth., September 1, 2001; 87(3): 421 - 428.
[Abstract] [Full Text] [PDF]
 |
 |
|
|
 |

|
 |
R. J. Chabot, F. di Michele, L. Prichep, and E. R. John
The Clinical Role of Computerized EEG in the Evaluation and Treatment
of Learning and Attention Disorders in Children and Adolescents
J Neuropsychiatry Clin Neurosci, May 1, 2001; 13(2): 171 - 186.
[Abstract] [Full Text] [PDF]
 |
 |
|
|
 |

|
 |

S. VALE
Medical urology.
Postgrad. Med. J., September 1, 2000; 76(899): 596 - 597.
[Full Text]
 |
 |
|
|
 |

|
 |
D. A. Hoffman, J. F. Lubar, R. W. Thatcher, M. B. Sterman, P. J. Rosenfeld, S.
Striefel, D. Trudeau, and S. Stockdale
Limitations of the American Academy of Neurology and American Clinical
Neurophysiology Society Paper on QEEG
J Neuropsychiatry Clin Neurosci, August 1, 1999; 11(3): 401 - 407.
[Full Text]
 |
|
|


![]()
![]() 0.05 level be replicated in each of these two samples, the probability that this would occur by chance would be approximately PxP, or 0.05x0.05, or 0.0025. If such a replication were required, false positives would seem rather unlikely. Such a high level of specificity is beyond the confidence level achieved by many routinely used clinical tests, such as mammograms, cervical screenings, or CT brain scans.34
0.05 level be replicated in each of these two samples, the probability that this would occur by chance would be approximately PxP, or 0.05x0.05, or 0.0025. If such a replication were required, false positives would seem rather unlikely. Such a high level of specificity is beyond the confidence level achieved by many routinely used clinical tests, such as mammograms, cervical screenings, or CT brain scans.34















